Membrane Fusion Events in the Sea Urchin
Work in emeritus profesor Poccia's lab was on the study of nuclear envelope formation by membrane fusion. In vitro work from the lab led to a pathway model involving a subset of isolated membrane vesicles (MV1) highly enriched in phosphorylated phosphatidylinositols (PI) and PLCγ . These bind to 2 sites on sperm chromatin initiating fusion leading to nuclear envelope formation and conferring spatial regulation on the process. Regulation occurs through an unknown GTPase and requires PI-3 kinase activity resulting in activation of PLCγ by a src family kinase SFK1. The PLCγ in turn hydrolyzes PIP2 in these vesicles leading to localized high concentrations of diacylglycerol which is a membrane destabilizing lipid and confers negative curvature required for formation of a fusion stalk. The confocal microscope has been used primarily to provide in vivo confirmation of the in vitro based model and to visualize the spatial aspects and kinetics of envelope formation in vitro.
Localization of PLCγ, SFK, and PIPK in sea urchin eggs and relationship to male pronuclear envelope formation
K. Han’06, J. Eastman’07, A. Cho’08, Sam Cutler’09
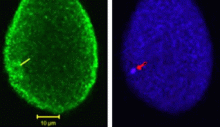
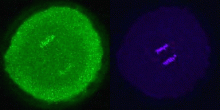
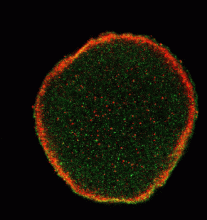
For the first time, a set of vesicles residing in the cortex of the egg highly enriched in PLCγ was demonstrated (Fig 1) using three different antibodies to PLCγ. These were correlated with the MV1 fraction. Their transient association with the male pronucleus following fertilization was documented (see Byrne et al., 2007). In addition, preliminary studies showed co-localization of PIP kinase (the enzyme that makes PIP2, the substrate of PLCγ. We have also demonstrated partial co-localization of SFK1 with MV1 (Fig 2). We are extending these studies to stages of mitosis (Fig 3) in the highly synchronized embryos to see if there are associations of these proteins with chromosomes during telophase as the mitotic nuclear envelope forms.
Fig 1 Fig 2 Fig 3
Contribution of the endoplasmic reticulum to the nuclear envelope
A. Cho’06, M. Dowicki’07, Burr Fong’08, Darienne Myers’09
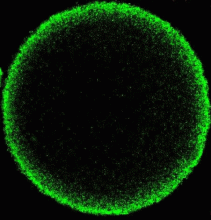
The outer nuclear membrane is continuous with the endoplasmic reticulum (ER). Two models exist for how the nuclear envelope forms. One suggests that the ER envelops the chromatin and then separates into inner and outer membranes; the other that vesicles bind to the chromatin and then fuse on its surface to create two membranes. We demonstrated that one set of vesicles contributing to the NE exists as such in vivo. The ER in the in vitro system however becomes vesiculated in preparing the cell-free system. This project takes advantage of the ability to label the endoplasmic reticulum in live cells by microinjection of oil saturated with a lipophilic fluorescent dye (DiIC16). Using this procedure we are able to clearly label the NEs of both male and female pronuclei (Fig 4,5) and should be able to visualize the formation of the male pronuclear envelope from ER components in live cells to determine the relative extent of envelopment vs vesiculation. Secondary labeling with DiOC6 allows visualization of vesicles in the live eggs.
Fig 4 Fig 5

Phospholipid probes of membrane vesicle populations in sea urchin eggs and embryos
Katerina Byanova’09
The unusual nature of MV1, with unprecedentedly high levels of phosphorylated PIs (determined by mass spectrometry) suggests that such vesicles could be revealed by probes recognizing these PIs. We have a tandem FYVE recombinant probe that recognizes PI-3 phosphate, MARCKs peptide that recognizes clustered PIP2 and a PH domain construct that recognizes PIP2 and PIP3. Preliminary work indicates that the FYVE probe colocalizes with MV1 in the cortex. (Fig 6)
Fig 6
Disposition of sperm membrane components following fertilization
M. Dowicki,’07 A. Cho’06
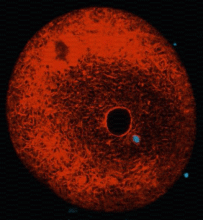
Remnants of the original sperm nuclear envelope do not disassemble after fertilization but appear to be incorporated into the newly forming male pronuclear envelope. To directly test this, we have labeled them with a relatively water soluble lipiphilic dye (diOC6) in live sperm and then fertilized eggs with these. The eggs were preloaded with a Hoechst dye that stains only the entering sperm nucleus making the nucleus easy to find quickly. Mitochondria and remnants can be found labeled in the egg whereas the rest of the nuclear envelope and plasma membrane disperse. We plan to use double lipid labeling procedures to observe the kinetics of fusion of the egg-derived membranes with the remnants during fusion. We are also exploring the use of the MARCKs peptide to label the remnants which are also enriched in PIP2. (Fig 7,8)
Fig 7 Fig 8
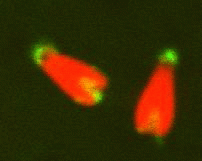
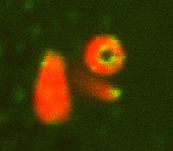
location of polar sperm nuclear envelope remnants (green stained with DiOC6)
Visualization of male pronuclear envelope formation in vitro
P. Murphy’08
The in vitro system is ideally suited for confocal localization and kinetic analysis of the in vitro reaction, since fluorescent dyes are available to separately label the different membrane fractions and the chromatin, and antibodies can also be used to tag specific protein markers. We will use the confocal microscope to evaluate purity of fractions and to follow binding and fusion kinetically in vitro. Quantification will be supplemented by fluorimetry and flow cytometry. FRET will determine membrane fusion. (Fig 9)
Fig 9
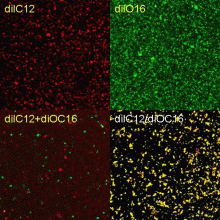
FUSION CONTROLS
Cytoplasmic extracts labeled with red and green lipophilic dyes separately (diIC12, DiOC16).
Vesicles were mixed together (DiIC12+DiOC16)or one set of vesicles was labeled simultaneously with both dyes(DiC12/DiOC16).
The latter probes are colocalized and exhibit FRET.
Monitoring ER contamination of isolated cortical granule preparations
F. Siddique’07
A second robust cell-free system derived from sea urchin eggs which exhibits membrane fusion is the cortical granule-plasma membrane complex. These complexes can be triggered to fuse by simple elevation of Ca++. We have begun to test if the model proposed for NE formation is more general and involved other fusions as well. To be sure that the system is not being complicated by Ca++ coming from the ER, we label the preparations with precipitates of DiI which only label adjacent membranes and thus spread only through ER similarly to the in vivo labeling described above and evaluate in the confocal microscope labeling non-ER membranes with