Dictyostelium discoideum RING - GFP Fusion Proteins
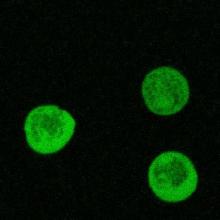
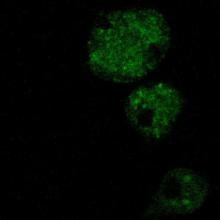
Emeritus Professor Ratner employed confocal microscopy to study the function of RING proteins in the amoeba, Dictyostelium discoideum. Proteins of this structural class are found in a wide range of eukaryotic organisms, including humans. They are thought to participate in ubiquitination reactions that tag particular intracellular proteins for degradation by the proteasome. Two of many Dictyostelium RING proteins are ZfaA and its highly homologous (96% amino acid identity) “Cousin.” We are investigating the intracellular location of these proteins by fusing their genes with that of jelly fish Green Fluorescent Protein (GFP), and then reintroducing the fused genes into amoebae. Initial confocal microscopy results suggest that GFP alone is distributed more or less uniformly throughout the Dictyostelium cytoplasm, while the DG17-GFP and Cousin-GFP fusion proteins are concentrated, at least in vegetative cells, in aggregates or vesicles of some sort, presumably reflecting the cellular regions in which these RING proteins act. All images are the work of Alyona Yermalovich (MHC '08).
GFP Positive Control AxDG17-GFP fusion